Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the concept of chemical bonding, the process by which atoms unite to form molecules and compounds.
ii. Identify and differentiate between the two primary types of chemical bonds: ionic and covalent bonds.
iii. Recognize the factors that determine the type of bond formed between atoms, including electronegativity differences and electron sharing tendencies.
iv. Describe the characteristics and properties of ionic and covalent bonds, understanding their distinct features.
v. Apply the knowledge of ionic and covalent bonding to explain the formation and properties of various compounds.
Introduction
The world around us is a captivating tapestry of diverse compounds, each held together by the intricate forces of chemical bonding. Understanding the mechanisms behind these bonds is crucial in unraveling the mysteries of chemistry and its profound implications for our lives.
i. Chemical Bonding: The Language of Atoms
Chemical bonding, the fundamental process by which atoms unite to form molecules and compounds, lies at the heart of chemistry. It is through the sharing or transfer of electrons that atoms achieve stability and give rise to the vast array of substances we encounter in our daily lives.
ii. Ionic Bonding: A Transfer of Power
Ionic bonding, a type of chemical bond, arises from the transfer of electrons from a metal atom, characterized by low electronegativity, to a nonmetal atom, with high electronegativity. This electron transfer results in the formation of oppositely charged ions, which attract each other due to electrostatic forces.
iii. Covalent Bonding: A Sharing Affair
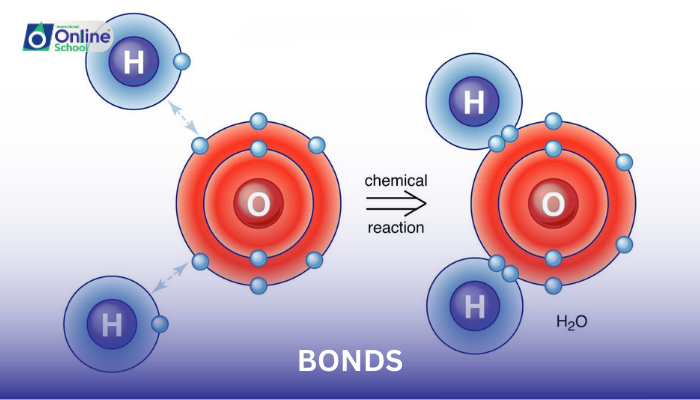
Covalent bonding, another type of chemical bond, involves the sharing of electrons between atoms. This electron sharing occurs when atoms have similar electronegativities or when an atom shares its electrons with a more electronegative atom to achieve a stable electron configuration.
iv. Factors Determining Bond Type
The type of bond formed between atoms is determined by several factors:
Electronegativity Difference: The greater the electronegativity difference between atoms, the more likely an ionic bond will form.
Electron Sharing Tendencies: Atoms with similar electronegativities tend to form covalent bonds due to their mutual attraction for electrons.
Valence Electron Configurations: The arrangement of valence electrons plays a role in determining whether an ionic or covalent bond will form.
Characteristics and Properties of Bonds
Ionic and covalent bonds exhibit distinct characteristics and properties:
Ionic Bonds: Ionic bonds are characterized by strong electrostatic forces, high melting and boiling points, and the formation of charged ions.
Covalent Bonds: Covalent bonds are characterized by the sharing of electrons, lower melting and boiling points, and the formation of nonpolar or polar molecules.
Examples of Ionic and Covalent Bonds
Sodium Chloride (NaCl): Sodium readily loses its single valence electron to chlorine, forming an ionic bond due to the significant electronegativity difference.
Water (H2O): Oxygen shares its valence electrons with two hydrogen atoms, forming covalent bonds due to similar electronegativities.
Carbon Dioxide (CO2): Carbon forms covalent bonds with two oxygen atoms, sharing electrons to achieve stable electron configurations.
Ionic and covalent bonding, the two primary types of chemical bonds, represent fundamental interactions that govern the formation and properties of a vast array of compounds. By comprehending the underlying principles of these bonds, we gain valuable insights into the intricate tapestry of chemical interactions, the behavior of matter, and the fascinating world around us.